Are you a quality-driven professional with a sharp eye for detail in pharmaceutical manufacturing? If you believe quality is built during production—not just tested at the end—this opportunity could be your next big career move.
Milan Laboratories India Private Limited is currently hiring an IPQA Officer for its manufacturing facility at Kamothe, Navi Mumbai. This role places you at the core of GMP compliance and patient safety. 💊✨
About Milan Laboratories India Pvt Ltd
Milan Laboratories is a well-established name in the Indian pharmaceutical industry, known for manufacturing high-quality generic medicines while maintaining strict regulatory compliance.
Working at Milan Laboratories means:
- Exposure to robust GMP systems
- Hands-on involvement in regulated manufacturing
- Career growth alongside experienced quality professionals
Job Location: Kamothe, Navi Mumbai 📍
Kamothe is one of Navi Mumbai’s most strategically developed nodes. Located near the Sion–Panvel Highway, it offers excellent connectivity from Mumbai, Navi Mumbai, and Panvel—making it a convenient work location with a balanced lifestyle. 🏙️
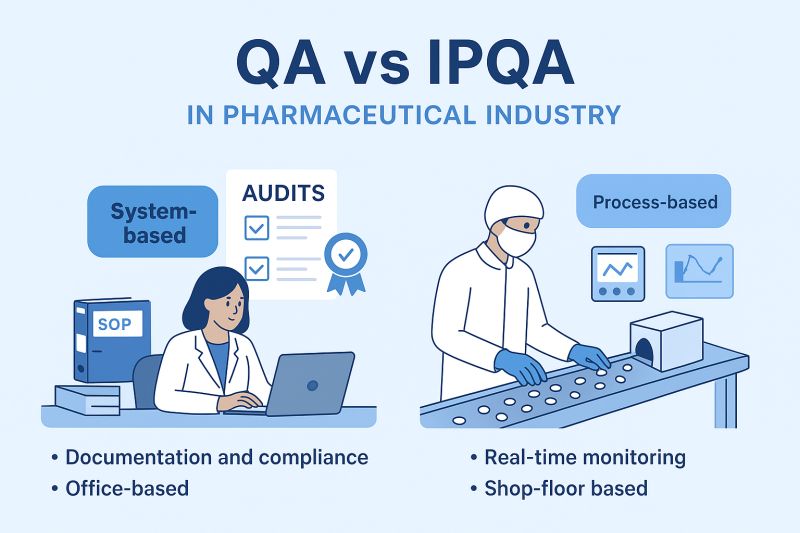
Role Overview: IPQA Officer (In-Process Quality Assurance) 🔬
The IPQA Officer is the shop-floor guardian of quality. Unlike laboratory QA roles, IPQA ensures that quality is embedded into the manufacturing and packing processes in real time.
Key Responsibilities
- In-Process Monitoring: Supervise manufacturing and packing activities to ensure SOP compliance 🧐
- GMP & SOP Compliance: Enforce adherence to GMP, SOPs, and regulatory guidelines
- Line Clearance: Perform line clearance between batches to avoid mix-ups and contamination 🧹
- In-Process Checks: Monitor tablet weight variation, seal integrity, labeling accuracy, and packing parameters
- Documentation Review: Real-time verification of BMRs and BPRs ✍️
- Deviation Handling: Support deviation management, investigations, and CAPA implementation
- Cross-Functional Coordination: Act as a quality bridge between Production and QA teams
Eligibility Criteria: Who Should Apply? 🎓
| Criteria | Requirement |
|---|---|
| Qualification | B.Pharm / M.Pharm / B.Sc (Science background) |
| Experience | 1–3 years in IPQA within pharma manufacturing |
| Knowledge | GMP, GLP, regulatory guidelines |
| Skills | Strong documentation, attention to detail, communication |
Pro Tip: Experience with WHO-GMP, PIC/S, or regulatory audits is a strong advantage—highlight it clearly on your CV.
Why Build Your Career in IPQA? 🚀
Choosing IPQA is a smart long-term move in pharmaceuticals:
- High Industry Demand: QA roles grow as regulations tighten globally
- Process Mastery: End-to-end exposure to pharmaceutical manufacturing
- Career Growth: Pathways to Senior QA, Quality Management, or Regulatory Affairs 📈
- Patient Impact: Your work directly safeguards patient health and product integrity
How to Apply 📩
Interested candidates should apply immediately.
- Email:
talentpool@milanlabs.com - Subject Line: Application for IPQA Officer – [Your Name] – [Years of Experience]
Ensure your resume clearly mentions:
- Shop-floor IPQA experience
- Documentation handling (BMR/BPR)
- GMP and deviation exposure
IPQA Interview Preparation Tips 💡
- Understand SOPs: Be ready to explain line clearance and in-process controls
- Know ALCOA+: Data integrity is critical—this topic often comes up
- Handling Conflicts: Prepare examples where you stopped production due to quality concerns 🤝
- Instrumentation Basics: Friability tester, disintegration apparatus, weighing balances