If you’re passionate about animal welfare, drug safety, and global healthcare compliance, this could be your next big move! Elanco (NYSE: ELAN), a global leader in animal health, is hiring an Analyst – Global Drug Pharmacovigilance (GDPS) in Bangalore, India.
This is not just a backend data role — it’s a frontline position in global drug safety, ensuring medicines for pets and farm animals remain safe, effective, and compliant worldwide.
🏢 About Elanco: “Food and Companionship Enriching Life”
Elanco operates with a powerful purpose — improving animal health to enhance food security and companionship worldwide. Their work spans:
- 🐶 Companion animal health
- 🐮 Livestock and farm animal medicines
- 🔬 Research-driven innovation
- 🌍 Global regulatory compliance
The company is deeply committed to diversity, inclusion, and employee growth, encouraging candidates from underrecognized groups to apply — even if they don’t meet 100% of the listed qualifications.
📋 Role Overview: Analyst – GDPS (Global Drug Pharmacovigilance)
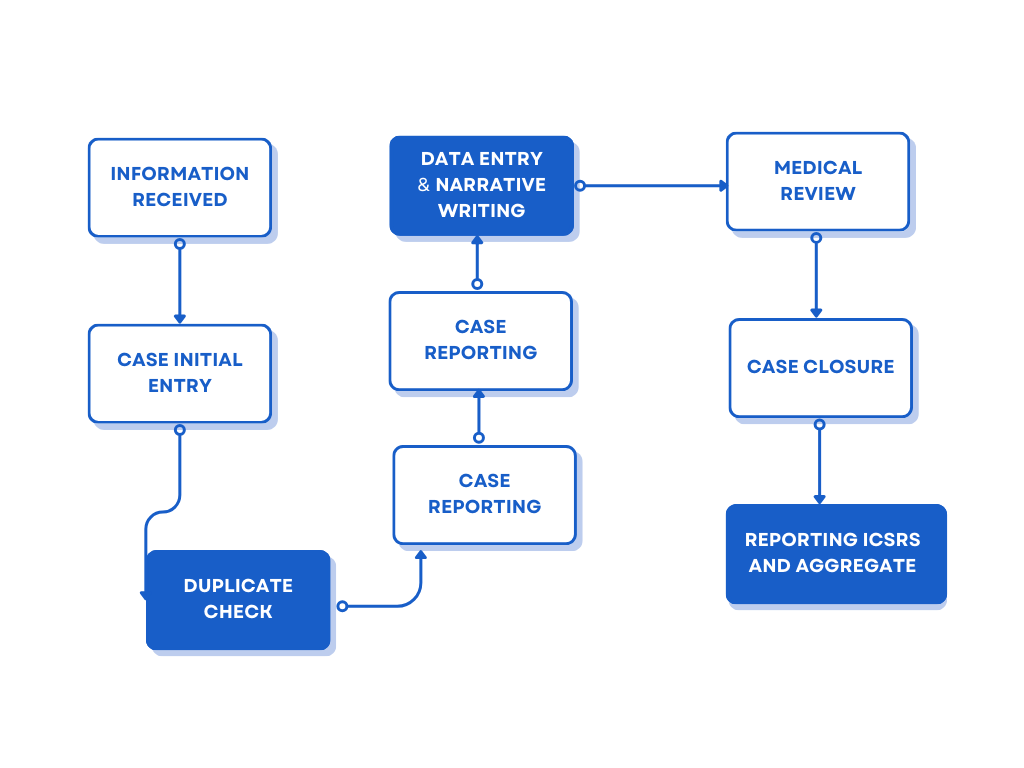
As a Pharmacovigilance (PV) Case Processing Analyst, you’ll be responsible for managing adverse event reports and ensuring regulatory compliance across global markets.
🔍 Key Responsibilities
- Case Management:
Entering and assessing adverse event reports in PV databases. - Medical Assessment:
Evaluating seriousness, expectedness, and causality. - Quality Control:
Validating safety data against source documents before regulatory submission. - Compliance & Timelines:
Ensuring reporting aligns with global authorities like the
U.S. Food and Drug Administration and
European Medicines Agency. - Follow-ups:
Identifying missing information and coordinating additional data collection.
This role demands precision, scientific understanding, and strong documentation skills — perfect for detail-oriented professionals.
🎓 Eligibility Criteria
✅ Minimum Qualifications
- 🩺 Veterinarians (Degree equivalent to US DVM; PV experience preferred but not mandatory)
- 🧪 Life Science Graduates with 0–2 years of Pharmacovigilance experience
⭐ Competitive Edge
- Familiarity with medical terminology
- Strong written & verbal communication skills
- Ability to manage multiple databases
- High attention to detail
- Adaptability during high-volume reporting periods
📊 Diversity & Pharma Workforce Trends
The life sciences industry is evolving rapidly:
- Women now represent 47–53% of the global life sciences workforce
- Around 25–30% hold C-suite roles
- India produces over 2.5 million science & engineering graduates annually, making Bangalore a major global pharmacovigilance hub 🌏
Elanco actively encourages applicants from diverse backgrounds, reinforcing that potential matters more than perfection.
📍 Why Bangalore?

Bangalore — India’s biotech and tech capital — offers:
- 🌐 Exposure to global regulatory frameworks
- 🤝 Collaborative and supportive teams
- 🏢 Work with a NYSE-listed global company
- ✈️ 0% travel requirement (stable routine)
It’s one of the strongest pharmacovigilance ecosystems in Asia.
💡 Why This Role is a Smart Career Move
Since you’re already writing pharma job posts regularly (and building authority in regulatory & PV niches), roles like this are perfect examples of:
- High-demand entry-level PV jobs
- Strong career progression into Drug Safety Associate, PV Scientist, Signal Detection roles
- Global exposure without relocation
If you’re targeting SEO traffic for pharma careers, this role hits strong keywords like:
Primary SEO Keywords:
Elanco Careers, Pharmacovigilance Jobs in Bangalore, Analyst GDPS Role, Animal Health Jobs India, PV Case Processing Jobs
Secondary Keywords:
Veterinary Jobs Bangalore, Drug Safety Analyst, Entry Level Pharmacovigilance 2026, Adverse Event Reporting Jobs, Elanco R0024602
🚀 How to Apply
Apply directly through Elanco’s official career portal:
🐾 Final Thought
Making animals’ lives better truly makes life better.
If you’re ready to build a career in Global Drug Safety, this could be your opportunity to step into the world of international pharmacovigilance with a respected global leader.