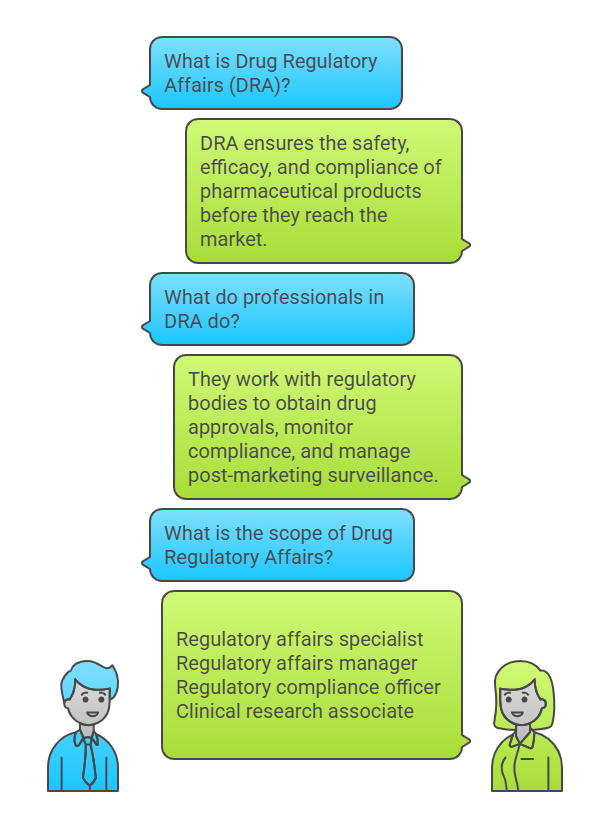
If you are passionate about clinical research, scientific writing, and patient safety, this opportunity at Meril could be the perfect next step in your career. Meril is inviting experienced clinical professionals to join Meril Academy Global – Clinical Affairs as a Clinical Executive in India.
This role offers hands-on exposure to clinical studies, regulatory documentation, publications, and collaboration with leading clinical experts—making it an excellent opportunity for professionals aiming to grow in the medical devices and healthcare industry. 🧬✨
🏥 About Meril & Meril Academy Global
Meril is a globally recognized healthcare organization known for innovation, quality, and patient-centric medical technologies. The company has built a strong reputation in the medical device space by delivering solutions backed by robust clinical evidence and ethical research practices.
Through Meril Academy Global, the organization focuses on advancing clinical excellence, education, and evidence-based practices across its product portfolio. The Clinical Affairs team plays a critical role in ensuring that all products are supported by high-quality clinical data and compliant documentation.
👩⚕️👨⚕️ Role Overview – Clinical Executive
The Clinical Executive role is designed for professionals with prior experience in clinical studies, trials, or observational research. In this position, you will support and manage clinical activities while contributing to scientific outputs and regulatory-ready documentation.
This role uniquely bridges clinical research, scientific writing, publication planning, and internal advisory support, making it ideal for candidates who enjoy both technical depth and cross-functional collaboration.
📋 Key Responsibilities
🔬 Clinical Studies & Research Management
As a Clinical Executive, you will support and oversee clinical studies by coordinating with:
- Investigators and clinical sites
- CROs and external partners
- Internal cross-functional teams
You will monitor study progress, review data quality, and ensure all activities comply with approved protocols, ethical guidelines, and regulatory requirements. Your contribution directly impacts patient safety and data integrity.
📝 Clinical Documentation & Protocol Development
Documentation is a core responsibility in this role. You will assist in developing, reviewing, and finalizing:
- Study protocols
- Case Report Forms (CRFs)
- Investigator Brochures
- Standard Operating Procedures (SOPs)
Accuracy, clarity, and compliance are critical, and you will ensure all documents meet internal quality standards and regulatory expectations.
📊 Clinical Reporting & Scientific Writing
For professionals interested in scientific communication, this role provides excellent exposure. You will contribute to:
- Clinical summaries and study reports
- Scientific documents for regulatory submissions
- Internal review and evidence-generation materials
You may also support data interpretation, helping translate clinical findings into meaningful, decision-ready insights.
📢 Publications & KOL Collaboration
The Clinical Affairs team works closely with Key Opinion Leaders (KOLs) and internal stakeholders to support scientific dissemination. Your involvement may include:
- Abstract and manuscript development
- Posters and conference presentations
- Publication planning activities
All outputs are developed ethically and transparently, aligned with global publication and regulatory standards.
🧠 Clinical Advisory Support
Beyond studies and documentation, you will act as a clinical resource for teams such as:
- Sales & Marketing
- Research & Development
- Quality & Medical Education
You may address clinical queries, support risk mitigation strategies, and provide evidence-based guidance on product use and best practices.
🎓 Qualifications & Experience
To be eligible for this role, candidates should have:
- Bachelor’s or Master’s degree in Biomedical Engineering, Life Sciences, Medical Research, or a related field
- Minimum 2 years of experience in clinical studies, trials, or observational research
- Prior exposure to medical devices or regulated healthcare environments (preferred)
🧩 Skills That Make You Stand Out
Meril is looking for professionals who bring:
- Hands-on experience in clinical research management
- Strong documentation and protocol development skills
- Confidence in clinical data interpretation and scientific writing
- Ability to collaborate with investigators, KOLs, and cross-functional teams
- High ethical standards and strong attention to detail
- Clear, professional English communication skills
📈 Performance Expectations
Success in this role is measured through:
- Timely and compliant execution of clinical studies
- Quality and accuracy of clinical documentation
- Contribution to scientific publications and evidence generation
- Responsiveness to internal and external clinical queries
- Strict adherence to ethical, patient safety, and regulatory standards
✉️ How to Apply
Interested candidates or referrals can reach out directly via email:
Or apply through the official LinkedIn job posting 👇
🔗 https://www.linkedin.com/jobs/view/4370009691
📌 Tip: Ensure your CV highlights clinical research experience, documentation skills, and exposure to regulated environments.
🌟 Final Thoughts
The Clinical Executive role at Meril is more than just a job—it’s an opportunity to contribute to impactful clinical research, strengthen scientific evidence, and support healthcare innovation at a global level.
If you are driven by accuracy, ethics, and scientific excellence, this role can be a powerful milestone in your Clinical Affairs career. 💡