Are you planning to build a long-term career in the pharmaceutical regulatory domain? Do you have early industry exposure and a growing interest in International Regulatory Affairs (IRA)? If yes, this opportunity could be the perfect next step in your professional journey. 🌱
A reputed pharmaceutical organization is currently hiring for the position of Executive – International Regulatory Affairs (IRA). This role is ideal for early-career professionals who want structured learning, global exposure, and steady growth in regulatory affairs.
🏢 About the Opportunity
International Regulatory Affairs plays a critical role in ensuring that pharmaceutical products meet global regulatory standards before entering international markets. Professionals working in this domain are responsible for regulatory documentation, submissions, and coordination across departments to ensure timely approvals.
As an Executive – IRA, you will gain hands-on exposure to:
- Regulatory documentation and compliance
- International filing processes
- Cross-functional coordination with QA, R&D, and manufacturing teams
This role helps build a strong foundation in global regulatory affairs, making it an excellent career-launching opportunity.
📌 Job Overview
Below are the key details of the opening:
- Job Title: Executive – International Regulatory Affairs (IRA)
- Industry: Pharmaceutical
- Experience Required: 6 to 12 months
- Preferred Background: IRA / Quality Assurance / R&D
- Employment Type: Full-time
This position is best suited for candidates who already understand basic pharmaceutical workflows and are looking to specialize in international regulatory operations.
🎯 Roles & Responsibilities
As an Executive in International Regulatory Affairs, your responsibilities will focus on regulatory documentation, compliance, and internal coordination. Key duties include:
- 📄 Preparing, reviewing, and maintaining regulatory documents
- 🌐 Supporting international regulatory submissions and filings
- 🔍 Ensuring documentation accuracy and compliance with global regulations
- 🤝 Coordinating with QA, R&D, production, and other internal teams
- 🗂️ Maintaining proper regulatory records as per guidelines
Attention to detail, consistency, and documentation discipline are essential for success in this role.
🧠 Required Skills & Qualifications
To perform well in this position, candidates should have:
- ✅ 6–12 months of experience in IRA, QA, or R&D within the pharma industry
- 📘 Strong interest in International Regulatory Affairs
- ✍️ Good documentation and compliance-handling skills
- 🗣️ Effective written and verbal communication abilities
- 🌟 A positive attitude with a willingness to learn and grow
Candidates who are organized, proactive, and adaptable to regulatory challenges will thrive in this role.
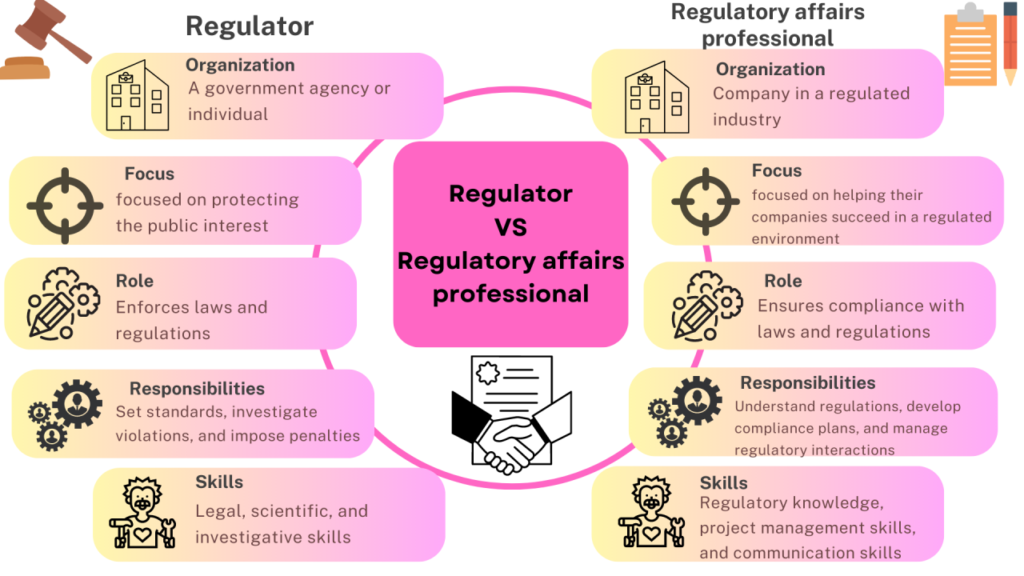
🎓 Who Should Apply?
This opportunity is ideal for:
- Early-career pharmaceutical professionals
- Candidates planning to shift from QA or R&D to Regulatory Affairs
- Professionals seeking exposure to international regulatory documentation
- Individuals aiming for long-term growth in global regulatory roles
If you are passionate about regulatory compliance and global pharma standards, this role offers an excellent career pathway.
🚀 Why Choose a Career in International Regulatory Affairs?
International Regulatory Affairs is one of the most stable and in-demand career paths in the pharmaceutical industry. Benefits include:
- 🌍 Exposure to global pharmaceutical markets
- 📈 Strong career growth and long-term stability
- 🧾 Development of high-value documentation and compliance expertise
- 🏆 Opportunity to work on international product approvals
Starting your regulatory career early gives you a competitive edge and prepares you for senior regulatory and compliance leadership roles.
📩 How to Apply
Interested candidates should send their updated CV to the email address below:
📧 sakhawat.imd@everestpharmabd.com
📝 Email Subject Line:
Application for Executive – IRA
Ensure your resume clearly highlights your relevant experience, documentation skills, and interest in International Regulatory Affairs.
✨ Final Words
The Executive – International Regulatory Affairs (IRA) role is a valuable opportunity for professionals looking to strengthen their position in the pharmaceutical regulatory domain. With structured exposure, learning opportunities, and global relevance, this role can help you grow into a skilled regulatory affairs professional.
If you meet the eligibility criteria and are serious about building a future in International Regulatory Affairs, apply today and take the next step in your pharma career. 💼🌟


